In a recent post I gave an overview about the role of Caspases in different human diseases and introduced tools to measure Caspase activity and inhibition by compounds in homogenous biochemical assays: Caspases as pharmaceutical targets – how to screen for inhibitors? Today I would like to give you an overview about a method and kits which allow to detect active Caspases in cells and which give an insight into the apoptotic status of the respective cells. Hence the effects of inducers of apoptosis can be investigated in living cells.
Fluorochrome Inhibitor of Caspases Method (FLICA)
The methodology used is based on the unique cell-permeable and non-cytotoxic reagents called the Fluorochrome Inhibitor of Caspases (FLICA). Once inside the cell, the FLICA™ inhibitor binds covalently to the active Caspase. For the FAM FLICA™ kits, which fluoresce green, a carboxyfluorescein-labeled fluoromethyl ketone peptide inhibitor of the specific caspase is used (EX:488nm/EM:520nm), compatible with most fluorescence microscopes or microplate readers. We can also offer a line of red FLICA Apoptosis Detection Kits that use sulforhodamine labeled
inhibitors.
Staining apoptotic cells with the FLICA™ kit can be completed within a few minutes. However, the FLICA™ kit is used with living cells, which require periodic maintenance and cultivation several days in advance. In addition, once the proper number of cells has been cultivated, time must be allotted for the induction process. The FLICA™ kit works with your current apoptosis protocols – induce apoptosis as you normally would and then label the cells with FLICA™.
Protocol of the method
- Culture cells to a density optimal for apoptosis induction according to your specific induction protocol, but not to exceed 106 cells/mL.

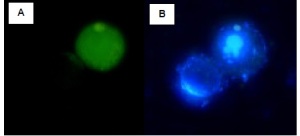
Jurkat cells were either treated with DMSO (negative, non-induced cells – left) or with staurosporine (apoptotic, induced cells – right) for 2 hours at 37°C. Cells were labeled with FAM-VAD-FMK solution for 60 minutes at 37°C. Samples were read on a 96-well fluorescence plate reader (Molecular Devices, Gemini XS) set at 490 nm excitation and 520 nm emission using a 495 nm cut-off filter. As the caspases became more active, indicating apoptosis, the amount of green fluorescence increased by over 500% in the induced Jurkat cells. - At the same time, culture a non-induced negative control cell population at the same density as the induced population for every labeling condition.
- Induce apoptosis following your protocol (such as treating Jurkat cells with 2 mg/ml camptothecin for 3 hours).
- Dilute the 10X wash buffer to 1X.
- Reconstitute the vial of lyophilized FLICA™ with DMSO to form the 150X FLICA™ stock concentrate.
- Dilute the 150X FLICA™ stock to the 30X working solution.
- Stain cells by adding the 30X FLICA™ solution.
- Incubate for 1 hour.
- Wash and spin cells.
- If desired, label cells with Hoechst 33342 stain.
- If desired, fix cells.
- Analyze data via microtiter plate fluorometry or fluorescence microscopy.
Which active Caspases can be detected?
Marker Gene Technologies offers kits for all Caspases involved in Apoptosis, being Caspase-3 and 7, Caspase-6, Caspase-8, and Caspase-9) and some other rather linked to inflammation (Caspase-1) or not yet clearly classified concerning the function (Caspase-2, Caspase 10).
Furthermore, we can offer poly Caspase assays which detect different active Caspases in cells.
And finally I would like to mention that the same technology can also be applied to Cathepsins, ubiquitous lysosomal proteases which play an important role in the turnover of intracellular proteins and extracellular proteins via endocytosis.
Should you have any questions or would like to share comments please use the form below.



