The transition from non-invasive phenotype to invasive phenotype of tumor cells marks the switch from a benign tumor to a more malignant form of cancer. Understanding the mechanisms underlying this hallmark event, which enables tumor cells to invade through Extracellular matrix, is critical for discovering pathways and new targets to develop anti-metastatic strategies. In a previous post, A 96-well Invasion Assay Compatible with High Content Screening, I introduced a technology called Oris™ assays, which employs exclusion zone technology to facilitate unambiguous monitoring of cell migration from the periphery into a central circular detection zone. This assay is a high-throughput assay, but compatible with adherent cells only. Today I’d like to introduce a new 3D Embedded Invasion Assay, compatible with both non-adherent and adherent cells.
Assay Overview
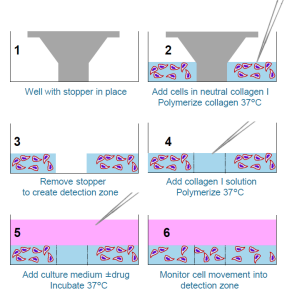
The Oris™ 3D Embedded Invasion Assay plates are provided with a Collagen I coating and with stoppers in the wells, ready for cell seeding:
Movement of cells through the collagen was assessed in multiple focal planes in 100 μm increments from the plate bottom up to 1 mm. Images were captured and ImageJ software was used to threshold stain intensity to distinguish between in-focus and out-of-focus cells, generating automated cell counts. Cells near the bottom of the plate are not used to quantify 3D migration.
Cells Migrate in 3D
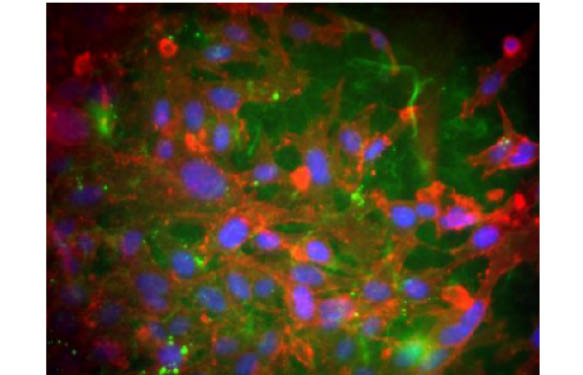
An Oris™ 3D Embedded Invasion Assay plate was seeded with 30,000 HT1080 cells per well and incubated for 5 d at 37°C. DAPI-stained images were collected and cells were counted using ImageJ.

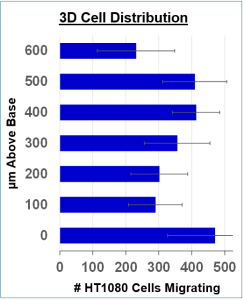
3D Cell Distribution
30,000 cells in 40 μL neutralized collagen I solution were seeded into each well of an Oris™ 3D Embedded Invasion Assay plate. Embedded cells were incubated at 37°C for 1 hour to allow the collagen to polymerize. Stoppers were removed and 10 μL of fresh neutralized collagen I was pipetted into the empty central column and allowed to polymerize at 37°C for 1 hour to create the detection zone matrix. Culture medium (100 μL per well) was then added and plates were incubated at 37°C in a 5% CO2 atmosphere for 5 days (HT1080).
3D Cell Distribution: Cells were fixed with glutaraldehyde and stained with DAPI, then imaged with a Zeiss Axio Observer Z.1 inverted microscope with automated stage, capturing images of each well at 0-600 μm above the plate bottom in 100 μm increments. Cell counts were determined using ImageJ to define the detection zone and to count infocus cells in each plane using a brightness threshold, averaging four wells per data point. The stack of 7 images to the right of the histogram shows ImageJ reconstructions of infocus cells at each height.
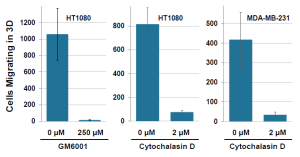
Cells Respond to Migration Inhibitors
MMP inhibitor GM6001 and actin polymerization inhibitor Cytochalasin D blocked 3D migration of HT1080 cells in the Oris™ 3D Embedded Invasion Assay. Cytochalasin D also blocked HT1080 cell migration in this assay:
Inhibition of cell migration: 30,000 cells were seeded per well in Oris™ 3D Embedded Invasion Assay plates. Culture medium +/- drug was added to the well after collagen in the central detection zone was allowed to polymerize. Cells were fixed, stained with DAPI and counted after 5 d (HT1080) or 7 d (MBA-MD-231) of incubation.
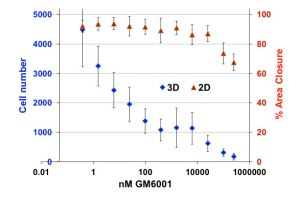
Cell movement in the exclusion zone is specifically inhibited by MMP Inhibitor GM6001
Matrix metalloproteinase (MMP) inhibitor GM6001 blocks movement of HT1080 cells through the collagen matrix in the Oris™ 3D Embedded Invasion Assay but not cell movement in the Oris™ Cell Migration Assay that monitors 2D migration across the plate surface:
30,000 cells were seeded per well in Oris™ 3D Embedded Invasion Assay plates (3D, blue) or Oris™ Cell Migration Assay (2D, red) plates. Culture medium ± drug was added to wells after collagen in the exclusion zone was allowed to polymerize (3D) or after stoppers were removed following cell adhesion (2D). After 1 day (2D) or 5 days (3D) cells were fixed and stained with DAPI (3D) or TRITC-Phalloidin (2D), then imaged on a Zeiss Axiovert Observer Z.1 microscope with automated stage. Images were analyzed using ImageJ. 3D invasion was quantified by counting cells in each of six focal planes at 0-500 µm above the plate bottom in 100-µm increments, summing cells in each plane for each well. 2D migration was quantified by measuring percent area closure in the exclusion zone. Data points are average of six wells ± standard deviation.
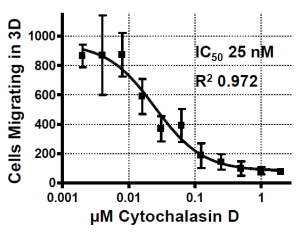
IC50 Measurement
Measurement of 50% inhibitory concentration (IC50) for cytochalasin D. HT1080 cells were seeded at 30,000 per well in the Oris™ 3D Embedded Cell Invasion Assay. Culture medium contained the indicated concentrations of cytochalasin D. Each data point represents four replicate wells ± standard deviation.
Conclusion
Oris™ assays employ exclusion zone technology to facilitate unambiguous monitoring of cell migration from the periphery into a central circular detection zone. No cell tracking systems are needed to determine whether cells moved. Oris™ 3D Embedded Cell Invasion Assay is compatible with both adherent and non-adherent cell types.