The U.S. Food and Drug Administration (FDA) has approved Velcade (Bortezomib) for the retreatment of adult patients with Multiple Myeloma who had previously responded to Velcade therapy and relapsed at least six months following completion of prior treatment. (1)
Bortezomib (marketed as Velcade by Millennium Pharmaceuticals Inc.) is a potent and selective Proteasome inhibitor. This di-peptide (formerly called PS-341) was the first therapeutic proteasome to be tested in humans and approved in the U.S. for treating Multiple Myeloma (2, 3). Besides Multiple Myeloma, Bortezomib was also cleared to treat patients with Mantle Cell Lymphoma.
The proteasome regulates protein expression and function by degradation of ubiquitylated proteins. By interacting with the catalytic site of the 26S proteasome, Bortezomib prevents pro-apoptotic factor degradation thus allowing the activation of apoptosis in malignant cells.
This shows that the importance of the Proteasome / Ubiquitin pathway and small bioactive molecules in Drug Discovery research programs and anti-cancer therapy arsenal.
References
- FDA Approves VELCADE® (bortezomib) Retreatment in Patients with Multiple Myeloma (August 2014), – Millennium (A Takeda Oncology Company).
- Kane R.C. et al. “Velcade®: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy” The Oncologist (2003), vol. 8 – no. 6, p508-513. doi: 10.1634/theoncologist.8-6-508.
- Velcade (bortezomib) is Approved for Initial Treatment of Patients with Multiple Myeloma (2008) – U.S. Food and Drug Administration (FDA)
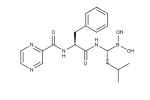
Bortezomib structure (Velcade®). Source: Focus Biomolecules.
See also
A selection of ultra-pure, cell permeable and potent Proteasome Inhibitors for R&D purposes:
- Aclacinomycin A
- Bortezomib
- Carfilzomib (CFZ – marketed under the trade name Kyprolis, Onyx Pharmaceuticals, Inc.)
- Celastrol
- Epoxomicin (BU4061T)
- Lactacystin
- MG-132
- NSC-687852/b-AP15
What about you?
Which Proteasome inhibitor are you looking for? Leave a comment on this post or share your thoughts with other readers!