PARPs (Poly ADP ribose polymerases) are found in the nucleus of the cell and they are involved in SSB repair (single-strand DNA breaks). PARP is known to bind damaged DNA through its N-terminal zinc finger domain. Subsequently it starts to synthesize a poly (ADP-ribose) chain which serves as a signal for other DNA-repairing enzymes.
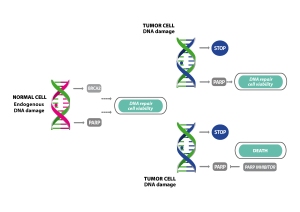
PARP inhibitors are considered to be promising candidates as anti cancer drugs (recently Olaparib, the first drug directed against PARP1, has been approved by the European commission). One of the reasons is that some tumors are more dependent on PARP than regular cells. These cancer cells are mutated in BRCA1 or BRCA2 – both genes which are involved in key DNA damage repair mechanisms. In healthy cells PARPs can function as a kind of back-up system and let the cells survive even without functional BRCA gene products. When PARPs are inhibited the cells do not possess any functional SSB repair mechanism anymore and are bound to die (Fig. 1; take a look at PARPs as cancer drug targets – first EC-approved drug to learn more about the concept of “synthetic lethality”).
Modes of action of PARP inhibitors
As PARPs have a clearly defined enzymatic activity, PARP inhibitors have been meant to decrease the activity of enzyme targets in the past. In 2012, a new mechanism following PARP inhibition has been described (1). Inhibition can take place by fixing PARPs at the sites of DNA damage, which results in a trapped PARP-DNA complex. These complexes are very toxic to cells because they totally block any DNA replication, an effect which can be used for anti cancer cell treatment.
PARPtrap assay to measure PARP1-DNA complexes
BPS BioSciences recently launched a new PARPtrap Assay Kit which is designed to measure PARP1-
formation in a high throughput screening assay using fluorescence polarization (FP). It is a 96-well format assay, which contains purified PARP1 enzyme, fluorescent labeled nicked DNA, and PARPtrap assay buffer for 100 enzyme reactions. The key to the PARPtrap Assay Kit is the fluorescent labeled nicked DNA. Without the Poly-ADP ribosylation reaction, PARP1 binds to the fluorescent labeled nicked DNA, resulting in the emission of highly polarized light. However, after autoribosylation of PARP1, the nicked DNA is dissociated from PARP1 and rotates freely, emitting less polarized light (Fig. 2).

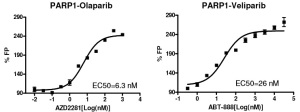
As soon as PARP1 is inhibited by specific compounds, the dissociation of the enzyme does not take place – in other words it is trapped on the DNA, thus the fluorescence polarization signal is high (see Fig. 3 – PARP1 inhibition by Olaparib and Veliparib). Thus the measurement of the trapping can be used as a way to detect the effects of potential PARP inhibitors.
Portfolio of active PARPs and PARP activity assays
Besides the new PARPtrap assay, a broad range of reliable active enzymes, PARP modulators or activity assays for most of the PARPs (and also tankyrases known so far) are now commercially available for in vitro applications making PARP-related research programs more effective.
Any questions? Are you planning screenings PARP inhibitor screenings? Leave your comments or get in touch below!
Reference:
(1) Murai, J. et al., Differential trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Research 72 (21): 5588–99 (2012)