Staying at the forefront of innovative cellular reprogramming technologies, ReproCELL’s Stemgent RNA Reprogramming portfolio is being expanded to include a cellular reprogramming kit using self-replicative RNA (srRNA) to reprogram both human blood outgrowth endothelial progenitor cells (EPCs) and human fibroblasts to generate clinically relevant iPS cells. The srRNA reprogramming of EPCs into iPS cells is the first demonstrated commercial application of RNA for the cellular reprogramming of a blood-derived cell type. The non-viral/non-DNA nature of RNA reprogramming leads to clinically relevant iPS cell lines that are suitable for GMP-compliant research studies.
Reprogramming methods
Of all the reprogramming methods, RNA-based iPSC approaches using Sendai virus (Fusaki et al., 2009), miRNAs, and mRNA transfection (Warren et al., 2010) avoid potential integration problems associated with DNA-based approaches and at this point in time appear inherently safer methods for future clinical applications. However, due to persistent Sendai virus replication in iPSC clones, this approach requires a negative selection step followed by one or more recloning steps from the single-cell level to isolate virus-free iPSCs. The latest breakthrough methodology published in the September 2010 issue of Cell Stem Cell utilizes modified synthetic mRNAs (00-0067) to reprogram human fibroblasts with high efficiency and no genomic alteration.
The non-DNA and nonintegrating, self-replicating VEE RNA approach has the potential to simplify the generation of hiPSCs for use in disease cell-modeling studies and eventual cell therapy applications. The StemRNA-SR Reprogramming Kit contains three essential component reagents for non-integrating reprogramming that have been functionally validated to support the generation of induced pluripotent stem (iPS) cell colonies from blood-outgrowth endothelial progenitor cells (EPCs).
 Key Benefits
Key Benefits
Flexible technology generates high quality human iPS cell lines from both blood samples and fibroblast lines
The reprogramming kit protocol has the flexibility to use genetically stable EPCs derived from fresh or frozen human peripheral and cord blood, or neonatal and adult human fibroblasts.
Reproducible StemRNA-SR kit protocol requires two transfections
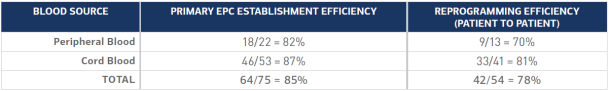
A two-transfection protocol includes one microRNA transfection to enhance reprogramming reproducibility and efficiency, and one srRNA transfection for OKSiM reprogramming factor delivery. Primary iPS cell colonies are generated in as few as three weeks with demonstrated patient-to-patient reprogramming efficiencies of ≥70% with the unmodified protocol (Table 1).
Virus and DNA-free StemRNA-SR kit extends RNA reprogramming technology for clinical research
Virus and DNA-free srRNA reprogramming of genetically stable EPCs combines easy clinical access to patient blood samples with integration-free RNA reprogramming technology. This facilitates the generation of clinically-relevant iPS cell lines.
Features
- Robust, validated protocol for reprogramming genetically stable EPCs derived from human peripheral or cord blood, and fibroblasts (neonatal & adult)
- Virus- and DNA-free OKSiM reprogramming factors using self-replicative RNA
- Virus- and DNA-free srRNA extends the product to clinical research
- Two-transfection protocol eliminates need for conditioned medium, co-transfections, small molecules and feeder cells
- iPS cell colonies generated from EPCs and fibroblasts in as few as 25 days
- Protocol accomodates 5 reprogramming wells in a 6-well plate format
Conclusion
Stemgent’s kit is the only commercially available application for RNA-mediated blood reprogramming being of non-viral and non-DNA nature and therefore makes it suitable for clinical applications by eliminating viral programs and the potential for DNA integration inherent to Sendai and Episomal reprogramming technologies. This technology saves researchers time by reducing the number of transfections from 12 to 2, providing ready-to-use iPS cell lines free of srRNA vector in 5 – 6 weeks.
Products
Stemgent StemRNA-SR Reprogramming Kit, 1 Kit, 00-0075
Components:
• OKSiM srRNA
• microRNA Reprogramming Cocktail
• B18R Recombinant Protein
NutriStemTM XF/FF Culture Medium, 500 mL, 01-0005
FGF-Basic, Human Recombinant (bFGF), 50 µg, 03-0002
StainAlive™ Tra-1-60 (Dylight™ 488), mouse anti-human, 100 μL, 09-0068



