Fluorescent technologies enable the measurement of protease activities and the screening of compounds influencing protease activities. Fluorescence Resonance Energy Transfer (FRET) but also TR-FRET, Q-FRET together with Time Resolved Fluorescence (TRF) and Fluorescence Lifetime (FLT) are popular assays, in which protease substrates occupy a central place. These assays are based on the specific recognition and cleavage of a peptide sequence (substrate) by the protease of interest. If the substrate is not optimally designed (or too short), experimental output can be unrelevant: low selectivity and specificity, high background, false negatives or positives…). In addition, the data can also be affected by the reaction buffer (pH, compound solvent…).This lack of relevant biological information is at the origin the emergence of “Next-Gen” fluorescent protease substrates.

Among these emerging new substrates, the EnSens® technology (by Enzium Inc.) is the first one to pull out of this gloomy situation. This platform is engineered to provide more relevant and sensitive Protease biological activity data.
The heart of the EnSens® platform is a recombinant substrate derived from an scFv-based protein. For a higher protease-selectivity, its design integrates the full protease recognition site sequence of the Protease of interest, with a structure allowing an easy swap of the protease recognition site. The long recognition sequence together with a modular “dumbbell-like” shape confers EnSens® substrates a higher stability for protease assays in a wide range of pH, salt, and solvent conditions.
These substrates are stable for at least six days in cell culture and the red fluorophore used is resistant to photobleaching for up to 48 hours. Interestingly, both of them are highly soluble and separate in the reaction. The fluorophore is thus constantly renewed in solution. In this platform, there is no energy transfer involved but just a mere “on-to-off” signal (Protease activity present or not), guaranteeing minimal self-quenching. An emission wavelength at 665 nm reduces interference from other assay components.
All these features make EnSens® a “2.0” Protease activity detection system in any fluorescent application including:
- Homogenous micro-well plate Protease assays with no washing steps. EnSens® can be rapidly adopted and integrated with any reagents and standard protocols with fluorescence detection equipments to directly convert RFU readouts to reaction velocity, Km, IC50 tests…

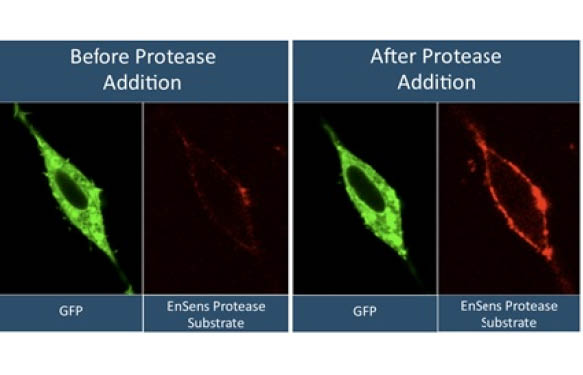
Comparison of EnSens® to FRET in HTS applications. Thrombin inhibitor IC50 curves were generated in HTS format using EnSens® vs. AMC-Thrombin substrates. Resulting IC50s were similar for both EnSens® and FRET substrates, demonstrating that EnSens® substrates can be easily used in HTS format for Protease inhibitor screening. Data generated by Dr. David Schultz,Molecular Screening Facility, WistarInstitute; Analyzed by Dr. CharalamposPapachristou, University of the Sciences of Philadelphia (USA). - Flow cytometry, Live-cell imaging including and High Content Screening.


Enzium’s approach is to make EnSens® available to the research community rapidly.
Highly validated Protease-specific assays for micro-well plate measurement and live-cell tracking (Factor Xa, Thrombin, Furin, ADAMs, , MMPs…) are regularly released while quick development of new substrates (even for up to 200 a.a. long recognition sequences) can be implemented through custom substrate service.
Would you like to know more about innovation in functional assays and Drug discovey tools? Stay tuned and subscribe to tebu-bio’s thematic e-newsletters!