Propelled into the spotlight by the COVID-19 pandemic, mRNA has become a biological entity of great interest in drug discovery – yet mRNA vaccines are just the promising beginning, leading to more and more discoveries and applications. mRNA demonstrates high potential to cure numerous other diseases, as we invite you to discover in this post – let’s take a look at a number of other mRNA based applications with active R&D, establishing mRNA beyond a current trend.
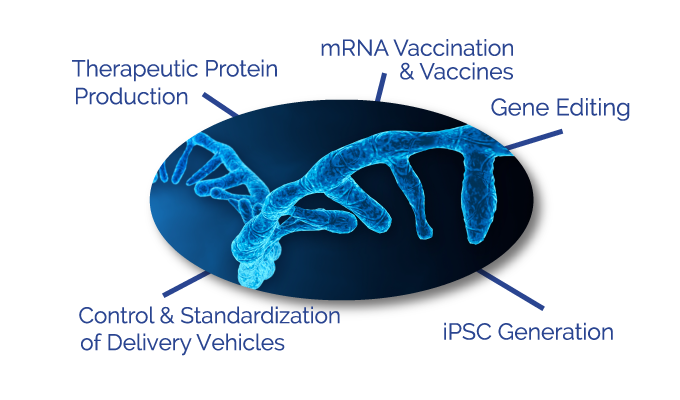
Active mRNA based applications
mRNA vaccines and vaccination
Research studies have shown that Nucleic acid based vaccines (pDNA and mRNA) 1,2 are a concrete alternative to “classical” live-based organisms with regards to safety profiles, specificity and induction of cell mediated Immunity3,4. Going deeper into the Nucleic Acid world, as a solutions for vaccines, mRNA have more advantages regarding pDNA:
- Safety profile – no risk of Genome integration and anti-DNA antibody induction
- Transient expression – Better control of antigen expression and less risk of tolerance induction due to long term antigen expression
- Dual purpose – Antigen expression and role as an adjuvant
- Capability to optimize mRNA sequence (Capping, Nucleotides..) – resulting in better stability, lower immunogenicity and higher level of expression
Based on all these features, Moderna and BioNtech have demonstrated that mRNA can be used for quick development (under 1 year) of new and efficient types of vaccines (low doses lead to high efficiency of vaccination). Furthermore, mRNA is easy to design and to upscale. It’s the ideal type of vaccine against episodic viruses such as influenza.
At research grade, OVA mRNA is one of the gold standard controls for vaccine development, and our partner Trilink, the worldwide leader in RNA technologies, have developed a fully optimized OVA mRNA with one of the most efficient capping technology on the market (Clean Cap) and Uridine modification to improve your vaccine development.
Control and standardization of delivery vehicles
Despite the promising potential of mRNA based vaccines, the main challenge remains optimization of its delivery into the cells. Low natural absorption of mRNA, negative charge density, instability… have pushed researchers to imperatively develop efficient delivery systems for high efficacy of mRNA solutions.
Even if many delivery vehicles are under active research and development2, such as exosomes, for now, it appears that the most efficient solution would be LNP (lipid nanoparticles), as we will discuss in our next RNA focus post in October.
But reliable control mRNA is the key point for these studies and the evaluation of your delivery model efficiency. Here at tebu-bio, we have a selection of fully optimized CleanCap® control mRNAs, such as the one coding for eGFP. Thanks to Trilink’s capping technology Clean Cap uridine and poly-A tail modifications, the Clean Cap control mRNA, mimicking fully processed mature mRNA, have become a real standard for delivery optimisation and quality controls.
To get started, we also provide a reference vehicle that works both in cell culture and in injection in mice, the mRNA-Fect combined with our booster reagent.
mRNA safety profile (non genome integration) as well as all the work performed on sequence optimisation to increase molecule stability, target expression, and reduce immunogenicity, have also opened the way to new mRNA based applications as described below.
Gene editing
With the advent of the CRISPR era, it’s now possible to knock-out a specific gene or to make a surgical modification in the genome. This is a big benefit for cell line engineering, for fine-tuning cell-based assays for drug screening and also for therapeutical purposes. By its principle of mechanism, the CRISPR-CAS9 system should be transient and without genome integration and off target effects in order to be optimal and clean.
All these points highlight mRNA as the perfect option for Cas9 expression and gene editing compared to pDNA or viral solutions. Several studies performed in mice4 have already shown the benefits of mRNA use in term of gene editing, but also with very low off-target effects.
For these reasons, we advise CAS9 coding mRNA in the optimized CleanCap® as your best solution for gene editing.
Therapeutic Protein expression
Recombinant proteins and antibodies have allowed significant breakthroughs in disease treatment due to their therapeutic applications:
- Protein replacement to restore the function of a single protein for rare monogenic diseases
- Cellular Immunomodulations where mRNA can be used by expressing transcription or growth factors
- Immunotherapies where mRNA encoded transcripts evoke specific immune responses against target cells, e.g., therapeutic antibodies5
But their use is also linked with some challenges such as pharmakocinetics (short half-life, fast clearance…) and manufacturing (Aggregates, long manufacturing time, animal care..). However, several studies2,5,6,7 have shown that improvement in immunogenicity, stability and target expression, has positioned mRNA as one as the best solutions for recombinant protein expression in therapeutical use.
As mRNA sequence optimization is pre-required for efficient recombinant protein expression, tebu-bio provides you with all the necessary tools and information to produce high quality and high yield mRNA.
Get 3 times more mRNA yield than with ARCA and ensuring capping near to 100%
N1mePseudo-UTP and 5mo-UTP,
to mention the most well-know today
iPSC generation
Despite great promises in regenerative medicine, induced Pluripotent Stem cell (iPSC) technology has major limitations before moving forward as a concrete clinical solution. Limitations are mainly based on the viral based reprogramming technology and the potential risk of genome integration and mutations.
It’s only in 2010 with the work of Warren et al, that modified mRNA appeared as a real alternative to virus for footprint-free iPSC generation. iPSC reprogramming studies performed on Fibroblasts8 and other cells types confirmed the fact that modified mRNA have all the necessary assets (low immunogenicity, high expression level of growth factor, transfection capabilities…) for efficient iPSC reprogramming and allow this technology to move to clinical applications.
Do you want to move to footprint-free reprogramming methods or improve your protocol? Have a look to our post How to produce capped mRNA with high yield | tebu-bio’s blog.
All the work and studies performed have shown all the potential, strengths and promises of mRNA for optimisation of many different types of applications, but more importantly in the development of new therapeutic solutions as shown by Moderna and BioNtech with the Covid-19 mRNA based vaccine.
Here at tebu-bio we bring you access to all the innovative tools (New Capping technology, modified Nucleotides, optimized catalog mRNA) and technical expertise you need to move forward in your mRNA based projects.
Lacking time ? Any specific needs for RNA? Not a problem…
Benefit from all the technical expertise of our laboratory and let them produce high quality RNA for you, through in-vitro transcription for RNA synthesis, ready for all your applications!
- Capped mRNA for drug development
- qPCR RNA control for sensitivity analysis
- qPCR RNA standard for absolute quantification
- many more…
How to get started? Just contact our Project Managers… Yes, that’s all – our lab will also take care of any plasmid preps and gene synthesis services!
References
(1) Deering RP et al, Expert Opin Drug Deliv. 2014. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines.
(2) Wadhwa et al, Pharmaceutics 2020 : Opportunities and Challenges in the Delivery of mRNA-Based Vaccines
(3) Chandler et al, Curr Opin Biotechnol 2020: Innate immune responses triggered by nucleic acids inspire the design of immunomodulatory nucleic acid nanoparticles (NANPs)
(4) Kowalski et al, Molecular Therapy 2019 : Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery
(5) Kaczmarek et al, Genome Med 2017: Advances in the delivery of RNA therapeutics: from concept to clinical reality.
(6) Beck et al, Molecular Cancer 2021: mRNA Therapeutics in cancer immunotherapy
(7) De Beuckelaer et al, Trends Mol. Med. 2017: Type I Interferons Modulate CD8+ T Cell Immunity to mRNA Vaccines.
(8) Kogut et al, Nature Communications 2018 : High-efficiency RNA-based reprogramming of human primary fibroblasts