Acid-Sensing Ion Channels (ASICs) are neuronal voltage-insensitive cationic channels which are activated by extracellular protons. They belong to the ENaC/Deg superfamily of ion channels. Up to now 6 members of the ASIC family have been identified: ASIC1 – ASIC 4, and the splice variants ASIC1a, 1b and 2a, 2b. The ASIC family members are trimeric and can be made up of different combinations of subunits. All ASICs are expressed in the peripheral nervous system. ASIC1a, 2a, 2b and 4 are expressed in the central nervous system. ASICs are promising drug targets for treating a wide variety of conditions linked to both the CNS and PNS especially implied in pain pathways.
Structure of Mambalgin-1

Mambalgin-1 (from Smartox, now available through tebu-bio) was initially isolated by Sylvie Diochot and collaborators from the venom of the black mamba (Dendroaspis polylepis polylepis, Fig. 1).
Mambalgin-1 belongs to the family of three-finger toxins (Fig. 2) and has no sequence/structural homology with either PcTx1 or APETx2. Mambalgin-1 differs from mambalgin-2 by one amino acid. Both have demonstrated a similar activity.
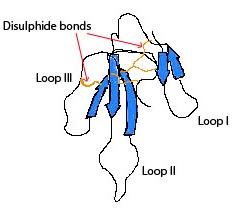
Mambalgin 1 – a blocker of ASIC1 channels implied in pain pathways
It has been shown that Mambalgin-1 is a potent and selective blocker of ASICs implied in pain pathways (1, 2). Mambalgin-1 rapidly and reversibly inhibits recombinant homomeric ASIC1a (IC50=55 nM) and heteromeric ASIC1a+ASIC2a (IC50=246 nM) or ASIC1a+ASIC2b channels (IC50=61 nM) but also human channels hASIC1b (IC50=192 nM) and hASIC1a+hASIC1b (IC50=72nM).
Mambalgin-1 has no effect on ASIC2a, ASIC3, ASIC1a+ASIC3 and ASIC1b+ASIC3 channels, as well as on TRPV1, P2X2, 5-HT3A, Nav1.8, Cav3.2 and Kv1.2 channels. Thus Mambalgin-1 can be used as a selective inhibitor for the above mentioned homomeric and heteromeric ASICs.
Interested in further information? Please contact me through the form below.
If you’d like an overview of the complete venomous toxins available, take a look to our special page: Synthetic peptide toxins ideal for studying ion channels
References:
(1) S, Dichot et al., Black mamba venom peptides target acid-sensing ion channels to abolish pain, Nature. 490 (7421), pages 552-5 (2012)
(2) Wen M., et al., Site-specific fluorescence spectrum detection and characterization of hASIC1a channels upon toxin mambalgin-1 binding in live mammalian cells. Chem Commun. 51 (38), pages 8153-6 (2015)


