Mouse Embryonic Fibroblasts (MEFs) are critical biological reagents in stem cell research. Used as “feeder cells” to maintain Embryonic Stem (ES) cells and induced Pluripotent Stem (iPS), the quality of MEFs strongly influences Stem cell culture conditions, and may react differently depending on culture conditions. In parallel, MEFs can also be reprogrammed into a variety of more mature differentiated functional cell types (cardiomyocytes, neurons…). During daily discussions with Stem cell biologists, I have been able to collect Frequently Asked Questions and technical tips related to MEFs.
As a start, and to avoid any major concerns in your cellular experimental designs, it’s best to use highly validated MEFs from popular sources. I personally recommend using qualified MEFs such as the GlobalStem MEFs. By using GlobalStem MEFs, you will be able to overcome the main problem areas seen during Stem cell cultures:
#1 – Low Cell Number / Low Viability
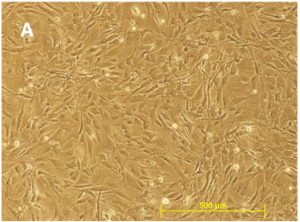
GlobalStem’s Quality Control includes determining cell number and viability of cells from a random sampling of vials for each lot. This means that after the cells have been cryopreserved, multiple vials are pulled from liquid nitrogen storage and cell counts are performed several times using Trypan Blue to determine cell number and viability.
If the values you obtain are below the specifications as found on the Certificate of Analysis, the best thing to do is to thaw and count another vial. If your values are low again, experimental optimization can be obtain by contacting cell biologists at tebu-bio who will assist you in thawing your MEFs.
#2 – Low Plating Efficiency
Plating efficiency is defined as the percentage of cells attached 24 hours after plating, relative to the number of cells originally plated. I recommend culturing MEF in GlobalStem’s dedicated media (ES-DMEM – cat. nr GSM-2001) supplemented with 15% ES-FBS (cat. nr GSM-6002).
All medium and reagents used should be warmed to 25–37°C before use. In addition, all activities must be carried out under aseptic culture conditions.
#2-1 – Recommended MEF thawing protocol:
1- Place the frozen vial into a 37°C water bath as soon as possible and retrieve the vial before the contents are completely thawed (1–2 minutes).
2- Immediately transfer the contents of the vial to a 15‐mL tube and dilute 1:10 with complete growth medium.
3- Spin the tube at 270 g for 5 minutes in order to pellet the cells.
4- Resuspend the pellet in complete growth medium and plate the cells to appropriate size tissue culture flask at the required density. Place culture flask in incubator overnight.
MEF should be plated 24 hours before being used as a feeder layer.
#2-2 – Recommended plating density:
There is a wide range of MEF plating densities used by researchers for mouse and human pluripotent stem cell culturing. The density depends on the ESC or iPSC lines you are culturing. I recommend that you use the feeder density previously used by the source lab from which you received the cell line; or the density determined through personal experience to be appropriate for your specific stem cell line. GlobalStem’s MEF have been successfully used to support undifferentiated pluripotent stem cells at densities ranging from 2 x 10E4 – 5.3 x 10E4 cells/cm2. These MEFs do not require gelatin coating of the plates prior to use. Using gelatin coated MEFs does not benefit or inhibit the ability of the MEFs to support pluripotent stem cells. MEFs adhere well to tissue culture plastic without coating the plastic with matrix proteins. I recommend not using gelatin with GlobalStem’s MEFs because it can introduce potential variability and toxicity into the cell culture system. It must be carefully screened before use.
Once solubilized, shelf life must be carefully monitored. It also adds an extra step and cost that is not required.
#3 – Problem Supporting ES Cells
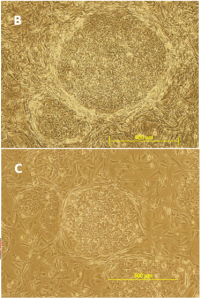
MEFs must be screened for the ability to support both human and mouse ES cells (this is the case with tebu-bio/GlobalStem MEFs). Feedback we have received over the years from our customers has proven that these MEF are able to support many different mouse and human ES and iPS cell lines. Our MEF users have also successfully used our MEF to support non-human primate ES cells. However, we do not claim that our MEF can support all ES lines. Our experience has shown us that some ES lines require more stringent conditions than others.
If you see an uncharacteristic degree of differentiation in your ES cultures after plating them on our MEF, please document your findings with images and contact me to share your experience.
#4 – Contamination
Each lot of MEF might be extensively tested for bacterial, fungal, and mycoplasma contamination. All tebu-bio/GlobalStem MEFs are also screened for a panel of 22 mouse pathogens. If you believe your cells are contaminated we recommend that you test each component of your cell culture system separately. Please keep in mind that contamination can be introduced by any component in your system – including the growth factors, plasticware, and human error.
After testing your components, thaw a fresh vial of our MEF and plate them into a new flask with medium and FBS that you have tested separately and found to be free of contamination. Leave MEF for 3-4 days in incubator. Examine the culture medium and cells for any contamination.
What about you?
As regular MEF users, you might have encountered technical challenges. You might like to share your tips and experimental optimizations. You might also be interested in additional information regarding Human Newborn Foreskin Fibroblasts (NuFF cells).




2 Responses
Hi Dear,
I was working on MEFS and suddenly it appeared that my cells got contaiminated by the yeast probably. These were in the 26th passage. so I want to ask if this passage no is too much for them or is it normal for MEFS.
Dear Nasir,
Thank you for your message.
Various parameters can be at the origin of such contaminations mainly with such a number of passages. My colleague Paola is going to contact you via e-mail to assist you there.
Regards,
Philippe