Protein phosphorylation analysis is more and more used for cell signaling studies. This post-translational modification regulates the function of numerous proteins in both physiological and disease-related conditions. Phospho-proteins can be easily monitored by using phospho-specific antibodies in various types of immuno-assays (Western blot, antibody array, phospho-ELISA…). However, data generated is reliable only if used from cell or tissue lysates that effectively reflect the phosphorylation state of initial samples. For this, optimal cell lysis buffers preserving phosphorylation status and protein stabilty are crucial.
3 tips to preserve phospho-proteins with home-made lysis buffers
As soon as the lysis buffer is added on samples, these are immediately disrupted with high risk of proteolysis and dephosphorylation. These drawbacks can be drastically reduced by:
- Leaving and handling samples on ice or at 4°C.
- Preparing extemporaneously lysis buffer
- Using “fresh” buffers containing detergents supplemented with protease and phosphatase inhibitors (RIPA buffer, NP40 buffer, protease and phosphatase inhibitors, cocktails of inhibitors…).
1 alternative: ready-to-dilute non-toxic lysis buffer optimized for phospho-protein isolation
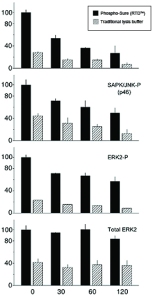
Alternative solutions have been specially developed for the extraction of phosphorylated proteins from neuronal, soft tissues types and cell lines (1, 2). Such ready-to-dilute (RTD) buffers, like the BioSensis’ Phospho-Sure™ Buffer, extract the phosphoproteins in a native state without the use of harsh detergents or oxidizers. It also helps maintain phosphoproteins and protect them from degradation better than traditional detergent based extraction buffers.
Phospho-Sure™ RTD is designed as both an isolation and extraction buffer.
For best results, the intact tissue should be also bathed in ice-cold Phospho-Sure™ buffer during excision of the tissue. Once the desired tissue piece is isolated, it may be transferred to a small volume of ice-cold Phospho-Sure™ for disruption.
Want to preserve phospho-proteins for Western blot and Antibody array protocols?
Phospho-Sure™ RTD™ Neuronal Cell & Soft Tissue Extraction Buffer is very convenient to use. Simply add sterile ddH2O directly to the powdered solution in the supplied bottle (100- or 200-ml format), swirl briefly to dissolve the chemicals and use. The material contains no preservatives so sterile technique should always be used.

1- Suneja S.K. et al. “Phospho-CREB and other phospho-proteins: improved recovery from brain tissue” (2006) Journal of Neuroscience Methods, Vol. 150 n° 2, 238-41. DOI: http://dx.doi.org/10.1016/j.jneumeth.2005.06.010
2- Mass E. at al. “Murine Creld1 Controls Cardiac Development through Activation of Calcineurin/NFATc1 Signaling” (2014) Developmental Cell, Vol. 28 n° 6, 711–726, DOI: http://dx.doi.org/10.1016/j.devcel.2014.02.012
Phospho-Sure™ users comments:
“Phospho-Sure™ Buffer helped to get strong, clear bands as published in the paper”.
“Very satisfied with the performance of the buffer to stabilize samples”.