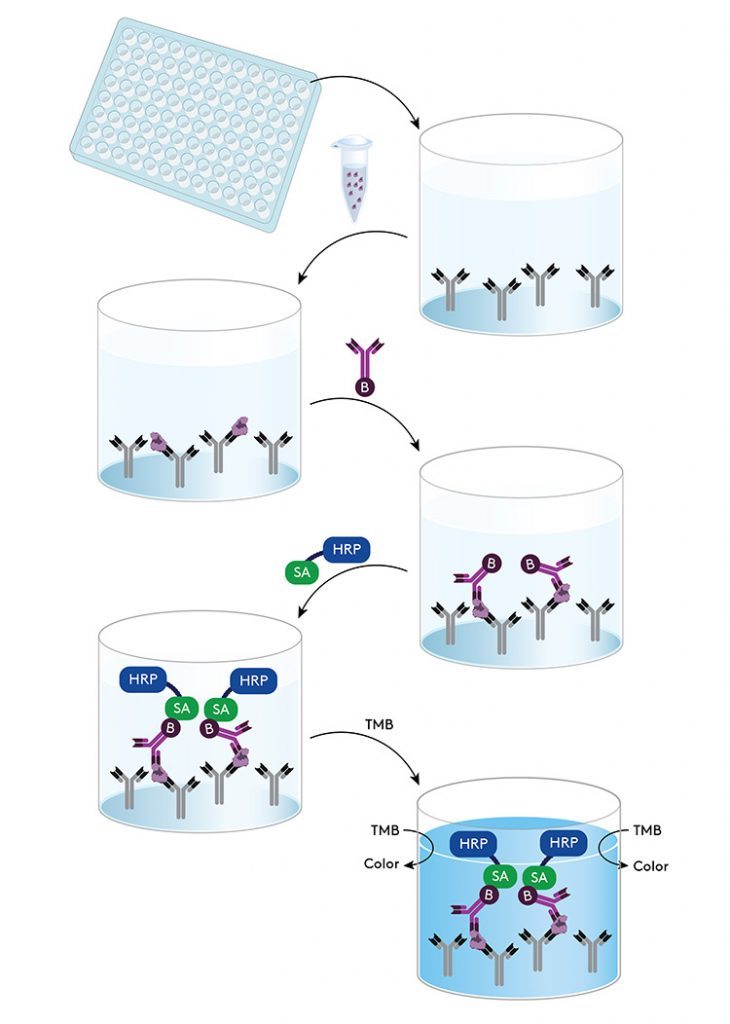
Expression systems – removing their contaminants
CHO, E. Coli and Pichia are useful expression systems for drug production. This can be for proteins, enzymes or antibody productions. Research and development for therapeutic perspectives requires a high level of quality controls. Such expression systems contain +1000 host cell proteins (HCPs). A major challenge in biological component production is to remove this HCP contamination. In order to monitor removal efficiency (during protein purification, as mentioned further on in this article), Canopy Biosciences have developed and optimised HCP detection kits based on the principle of the sandwich ELISA test as illustrated below.

High sensitivity detection and quantification of the HCP
The assay uses a standard for quantification. Thus, these sandwich ELISA tests are highly sensitive and provide concentrations of HCP. They can be used during the protein purification process in order to monitor and optimize that step of the bioproduction workflow.

There is a specific kit for each expression system: CHO, E. Coli and Pichia. For residual protein A in antibody production there is also a dedicated kit. Each kit allows quantification of up to 24 samples.
Of course, these HCP ELISA tests must be highly reproducible. Thus, variation coefficients are especially low as illustrated below with the CHO version.
What are the advantages of these Canopy HCP detection kits vs standard kits?
- 25% more coverage (CHO HCP antibody coverage is 251/348= 72%)
- 5 to 10 times more sensitive (contact me, I’ll be pleased to go into more detail)
- Lower lot-to-lot variations


Since the technology is based on an ELISA test, the quantification can be done using our tips and analysis tool available to download here. If you have any questions or would like to know more, just leave any comments below and I’ll be pleased to answer!