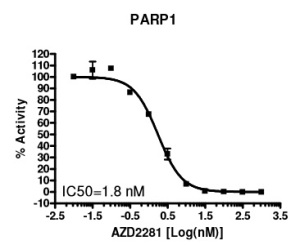
In December 2014, the European commission approved the first drug directed against a member of the PARP familiy (Poly ADP-Ribose Polymerases). Olaparib, a drug against ovarian cancer, has been developed by AstraZeneca and might become an important medicine very soon. Olaparib blocks PARP1 (see the Figure below showing the inhibitory effect of Olaparib on PARP1 measured with the PARP1 Chemiluminescent Activity Assay), an enzyme involved in cell repair, and is designed for ovarian cancer patients with certain hereditary gene mutations. It also has promise in treating other cancers, including breast and gastric tumors, opening up a substantial market opportunity. Olaparib is able to stop cancer cell growth in patients carrying inherited faults in the BRCA1 or BRCA2 genes (BReast CAncer 1 and 2 genes).
Synthetic lethality approach
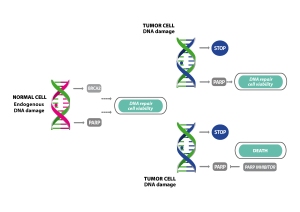
The principle of the Olaparib effect is known as “synthetic lethality”. Tumors which are caused by mutated BRCA1 or BRCA2 genes have problems with one of the key DNA damage repair mechanisms. PARPs can function as a kind of back-up system and let the cells survive even without functional BRCA gene products. As soon as PARP1 can be efficiently inhibited (e.g. by Olaparib) the cancer cells do not have any way of repairing damaged DNA and hence they die.
In addition to their use in cancer therapy and ongoing efforts to develop more anti cancer drugs, PARP inhibitors are considered a potential treatment for acute life-threatening diseases such as stroke, neurogenerative diseases, and myocardial infarction.
Tools to screen for PARP inhibitors
To help to accelerate the development of PARP inhibitors, BPS Biosciences has developed a broad portfolio of PARP related products and kits, which I’d like to present here.
- First of all, check out the most complete PARP isozyme portfolio available anywhere! All these enzymes have been proved to be biologically active, and you can use them to set up inhibitor screening assays.
- To avoid optimizing PARP assays yourself, you can also go for ready-to-use inhibitor screening assays for PARPs, such as PARP1 , 2, 3, 6, 7, 10, 11, 14, and 15 as well as for Tankyrase 1 and 2.
- And finally, you can also call upon a broad range of PARP modulators as reference compounds including Olaparib.
Do you have any needs in terms of PARP inhibitor screening, or any comments on the contents of this article? Or are you interested in outsourcing PARP inhibitor screenings?
I’ll be happy to respond to your feedback through the form below.